- -₴428.05
LifeVac, anti-choking device for airway clearance, Travel Kit, with carrying bag
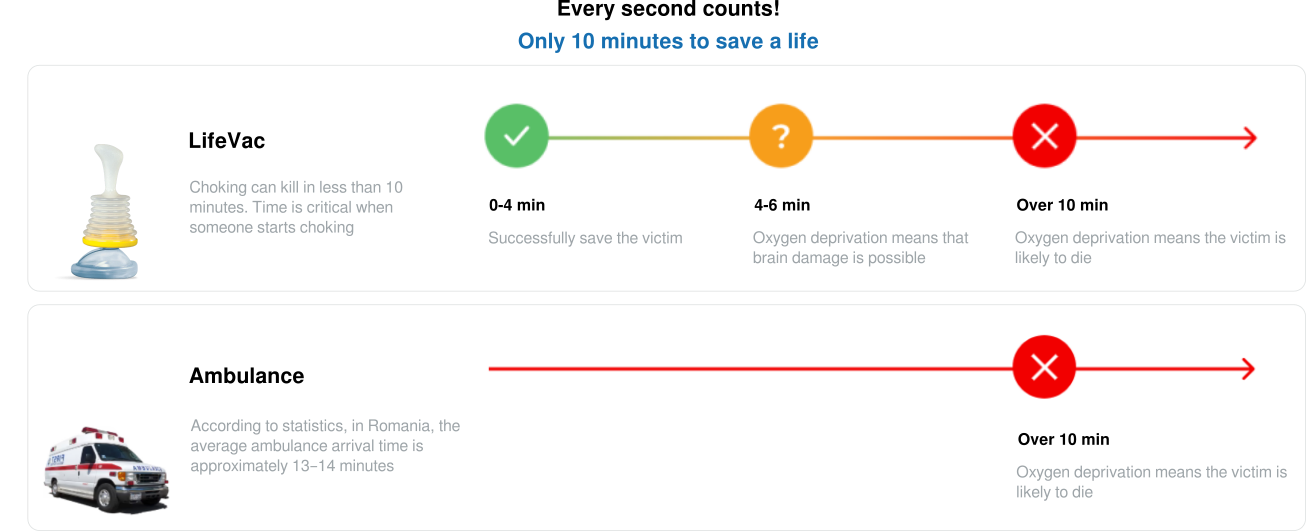
LifeVac is a manual, non-invasive, single-use device designed to intervene in cases of airway obstruction when standard clearing maneuvers have failed. The negative pressure generated by the LifeVac suction is up to three times stronger than the most effective abdominal thrusts recorded. The suction duration is extremely short, making LifeVac a safe and effective device.
LifeVac can be used on children weighing at least 10 kg, using the pediatric mask. However, there have been cases where the device was successfully used even on infants as young as 3 weeks old. The adult mask is intended for adults and elderly individuals. Both masks – pediatric and adult – are included in every Home Kit and Travel Kit.
The LifeVac device is equipped with an innovative one-way valve that prevents air from entering the airway during the push motion, thus preventing accidental pushing of the obstructing object deeper into the trachea.
All you need to do is place the mask over the casualty’s mouth and nose, push to create suction, and then pull to remove the obstruction. Thanks to the very short suction duration, LifeVac is safe and effective to use.
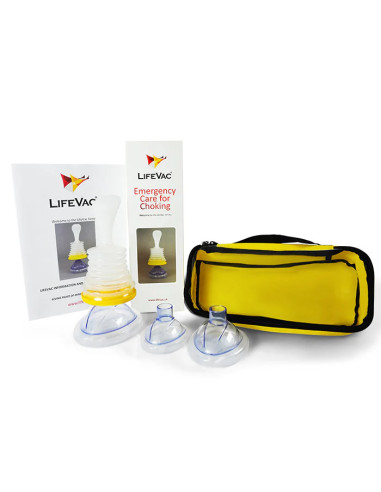
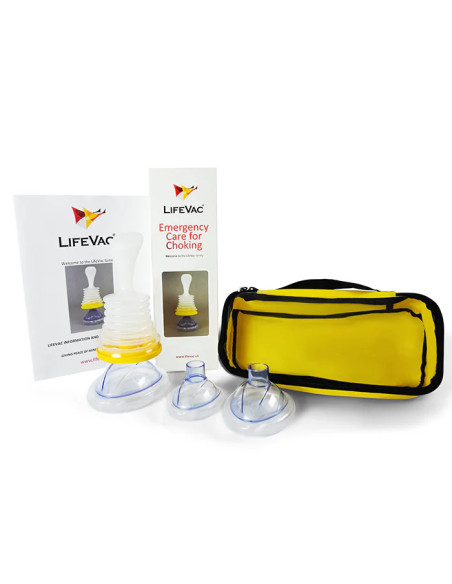
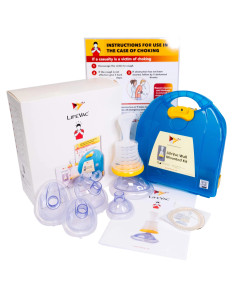
Package contents
- 1 x LifeVac device
- 4 x Masks (pediatric, child, adult, training)
- 1 x Instruction manual
- 1 x Carry bag
Certifications
LifeVac is a Class I medical device, CE and UKCA marked, compliant with Regulation (EU) 2017/745 (MDR). It is manufactured in the European Union following ISO 13485–certified procedures, and each unit is individually tested to ensure functionality and safety before leaving the factory. LifeVac is approved or registered with the FDA, MHRA, TGA, and other international regulatory authorities. These things may not mean much to most of us, but they guarantee that no compromises were made in the design and manufacturing of this device.
Certifications and regulatory compliance
The LifeVac device is a certified and rigorously tested Class I medical device, developed to meet the highest international standards for safety and performance. Its certifications and regulatory approvals include:
CE Marked
LifeVac is CE marked in accordance with the European Medical Device Regulation (EU) 2017/745 (MDR), ensuring compliance with essential health and safety requirements for medical devices in the EU.
ANMDMR Registered
LifeVac is officially registered with the Romanian National Agency for Medicines and Medical Devices (ANMDMR) as a Class I non-invasive medical device, authorized for sale and use throughout Romania.
ISO 13485 Certified
Manufactured in an ISO 13485 certified facility, guaranteeing a quality management system that meets strict international standards for the design and production of medical devices.
MHRA Registered (UK)
LifeVac is registered with the Medicines and Healthcare products Regulatory Agency (MHRA) for use in the United Kingdom.
FDA Registered (USA)
The device is registered with the U.S. Food and Drug Administration (FDA) under Establishment Registration guidelines.